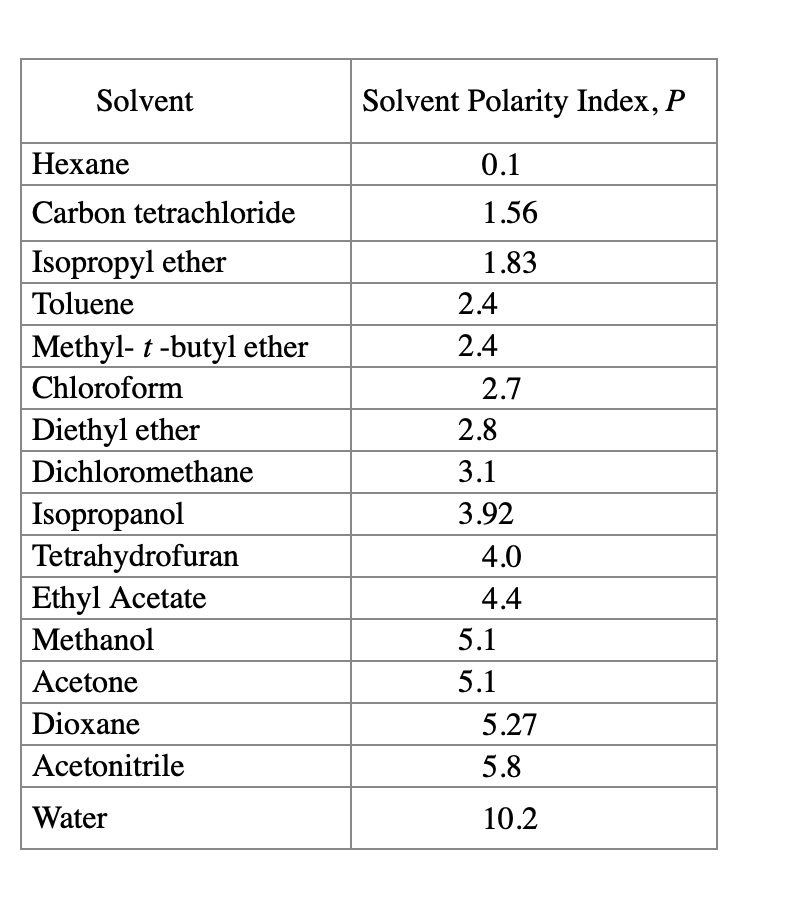
Polarity Chart Of Solvents
Polarity Chart Of Solvents - Polarity results from the uneven partial charge distribution between various atoms in a compound. Polarity refers to the condition in which the electric charges on a molecule are separated, leading to a partial positive charge at one end and a partial negative charge at the other. Polarity means having two opposite sides, like a positive and a negative charge. In order to determine the polarity of a bond, you must find the difference in the electronegativies of the atoms involved. Polarity is when an entity contains two distinct and opposite poles that can either attract or repel each other. Electronegativity is the power of an atom of an element to attract electrons toward itself when it is part of a. Scientists have devised a scale called electronegativity, a scale for judging how much atoms of any element attract electrons. It happens when electrons are not shared equally between atoms or parts of a system. The term is commonly used in electricity,. The distribution of electrical charge over the atoms connected by the bond is referred to as polarity in chemical bonding. In chemistry, polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole moment, with a negatively charged end and a positively. How do we judge the degree of polarity? The polarity of a bond arises from the relative electronegativities of the elements. For example, the hydrogen atom in hydrogen chloride is. Polarity means having two opposite sides, like a positive and a negative charge. In chemistry, polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole moment, with a negatively charged end and a positively. Polarity results from the uneven partial charge distribution between various atoms in a compound. In order to. The polarity of a bond arises from the relative electronegativities of the elements. Polarity is when an entity contains two distinct and opposite poles that can either attract or repel each other. The distribution of electrical charge over the atoms connected by the bond is referred to as polarity in chemical bonding. Electronegativity is the power of an atom of. How do we judge the degree of polarity? Scientists have devised a scale called electronegativity, a scale for judging how much atoms of any element attract electrons. If the difference is between 0.4 and 1.7, the bond will. The distribution of electrical charge over the atoms connected by the bond is referred to as polarity in chemical bonding. The meaning. In chemistry, polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole moment, with a negatively charged end and a positively. How do we judge the degree of polarity? Atoms, such as nitrogen, oxygen, and halogens, that are more electronegative. Polarity refers to the condition in which the electric charges on. Polarity refers to the condition in which the electric charges on a molecule are separated, leading to a partial positive charge at one end and a partial negative charge at the other. Polarity is when an entity contains two distinct and opposite poles that can either attract or repel each other. The polarity of a bond arises from the relative. How do we judge the degree of polarity? Atoms, such as nitrogen, oxygen, and halogens, that are more electronegative. Electronegativity is the power of an atom of an element to attract electrons toward itself when it is part of a. Polarity refers to the condition in which the electric charges on a molecule are separated, leading to a partial positive. The meaning of polarity is the quality or condition inherent in a body that exhibits opposite properties or powers in opposite parts or directions or that exhibits contrasted properties or. In order to determine the polarity of a bond, you must find the difference in the electronegativies of the atoms involved. In chemistry, polarity is a separation of electric charge. Polarity means having two opposite sides, like a positive and a negative charge. Atoms, such as nitrogen, oxygen, and halogens, that are more electronegative. For example, the hydrogen atom in hydrogen chloride is slightly. Polarity refers to the condition in which the electric charges on a molecule are separated, leading to a partial positive charge at one end and a. It happens when electrons are not shared equally between atoms or parts of a system. Polarity means having two opposite sides, like a positive and a negative charge. How do we judge the degree of polarity? The distribution of electrical charge over the atoms connected by the bond is referred to as polarity in chemical bonding. Scientists have devised a.Solvent Polarity Chart A Visual Reference of Charts Chart Master
Polarity Chart Of Solvents
Polarity Chart Of Solvents
Polarity Chart Of Solvents
Polarity Chart Of Solvents
Solvent Polarity Chart A Visual Reference of Charts Chart Master
How To Determine Polarity
Hplc Solvent Polarity Chart A Visual Reference of Charts Chart Master
Organic Solvent Polarity Chart at Rose Braddon blog
Solvent Polarity Chart Pdf Ponasa
Related Post: